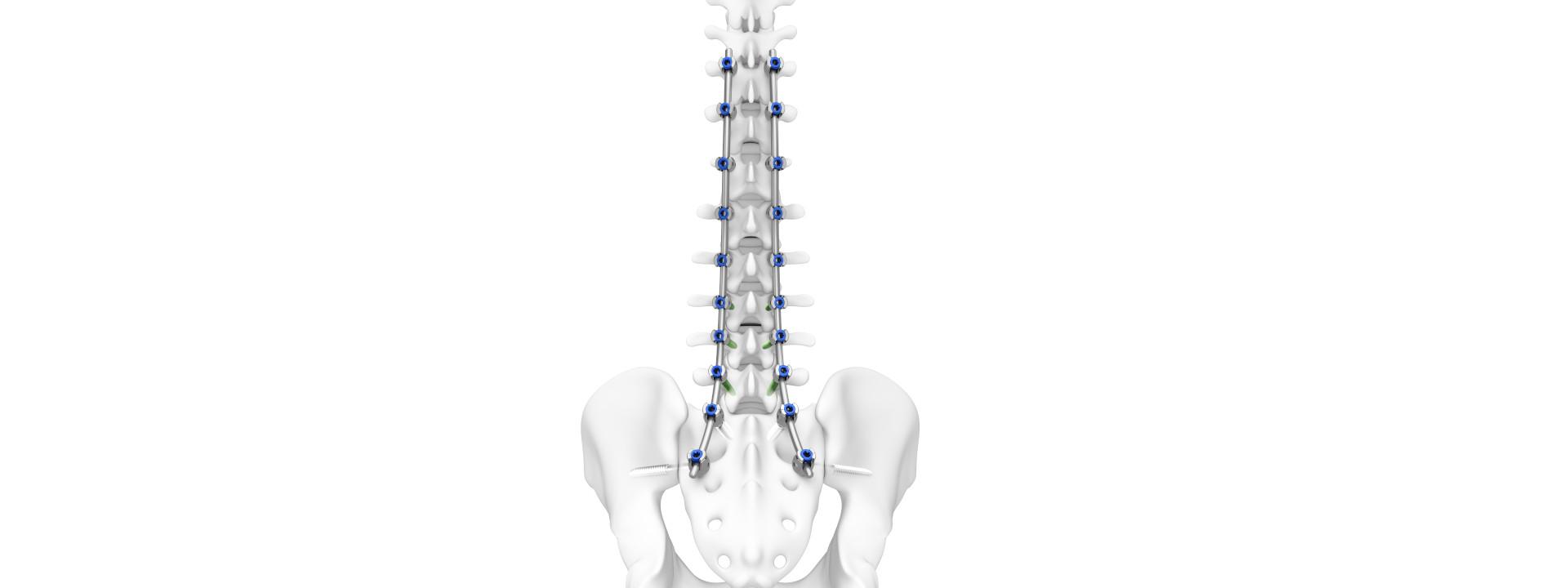

DePuy Synthes a Johnson & Johnson orthopaedics firm has been awarded US Meals and Drug Administration (FDA) 510(okay) clearance for its thoracolumbar implant system, TriATLIS Backbone System, and its Navigation Enabled Devices.
The TriATLIS Backbone System is a thoracolumbar pedicle screw system with a portfolio of devices appropriate with DePuy Synthes’ enabling applied sciences. It’s designed to help surgeons treating spinal points comparable to inclusive of degenerative, tumour, trauma, and deformity pathologies.
DePuy Synthes claims spinal deformity ailments impacts 32% of younger adults and 68% of adults over the age of 65. And with an ageing populations surgeons are seeing a wider scope of deformities that require spinal fusion devices.
Daniel Sciubba Professor of Neurosurgery at Northwell Well being/Hofstra praised the portfolio of implants from TriATLIS he mentioned: “In relation to treating advanced spinal pathologies, every affected person is completely different, and every case requires its personal distinctive remedy choices.”
Following the clearance, the TriATLIS Backbone System will likely be out there for the US market in 2024.
Based on GlobalData, the surgical sutures market is rising at 3.4% yearly to be price $4.5 billion by 2033, up from a price of $3 billion in 2022.
Entry probably the most complete Firm Profiles
in the marketplace, powered by GlobalData. Save hours of analysis. Acquire aggressive edge.

Firm Profile – free
pattern
Thanks!
Your obtain e-mail will arrive shortly
We’re assured concerning the
distinctive
high quality of our Firm Profiles. Nevertheless, we would like you to take advantage of
helpful
resolution for your online business, so we provide a free pattern which you can obtain by
submitting the beneath type
By GlobalData
In August DePuy Synthes secured FDA 510(okay) Clearance for its INHANCE Shoulder System and 510(okay) Clearance for MAXFRAME Multi-Axial Correction System.
Trending Merchandise










![[2025 Upgrade] Aotedor 30 Miles Long Travel Range, Electric Wheelchair for Adults Power Wheelchairs Lightweight Foldable All Terrain Motorized Wheelchair for Seniors Compact Portable Airline Approved](https://m.media-amazon.com/images/I/51vZJPDMrOL._SS300_.jpg)
